LEDs in Saline Solution
Experiment number : 1773
Goal of experiment
The goal of this experiment is to show the conductivity of liquids.
Theory
Acid, base and salt solutions that conduct electric current are called electrolytes. Their conductivity is caused by the electrolytic dissociation generating positive and negative ions. Electric field that is generated in the electrolyte between the cathode and anode, acts on the ions by electrical forces and causes them to move orderly – electric current is generated. The substance changes induced by the current passing through the electrolyte that occur at the electrodes are called electrolysis.
Voltage between the terminals of LED is proportional to the distance between the two ends and orientation relative to the electrodes – resp. the potential difference between the ends. The most shining LEDs are those with terminals oriented parallel to the electric field intensity and in the direction of a correct polarity (LED cathode nearer the negative pole of the source).
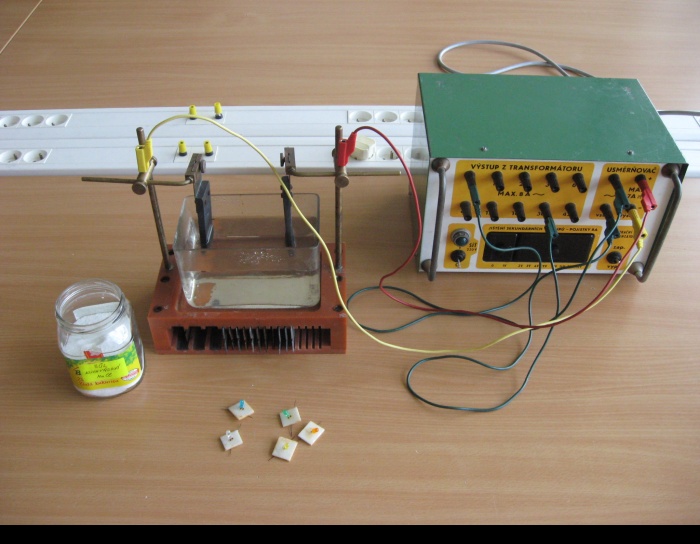
Tools
Procedure
- Spread the LED terminals apart and place them on small and light plates.
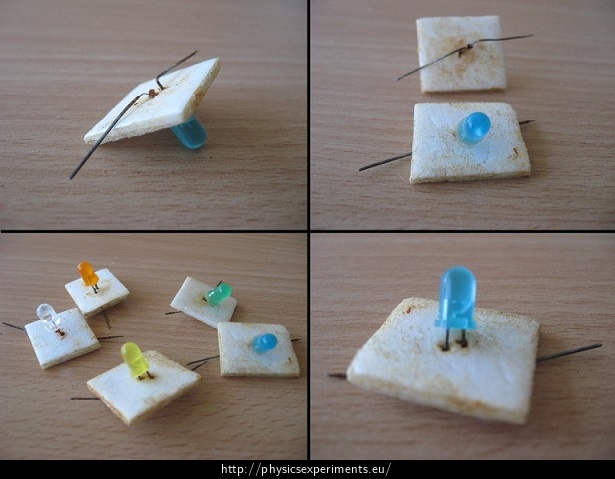
- Pour a concentrated salt solution into the container, insert the electrodes and connect them to the voltage source.
- Turn the voltage source on and gradually increase the voltage.
- If we insert the LED into the electrolyte which is under voltage, the LED lights up according to the orientation of its terminals to the intensity of the generated electric field.
Sample result
The video below illustrates a possible preformation of the experiment.
Technical notes
- It is possible to substitute the electrodes with an aluminium foil that covers two opposite walls of the container.
- It is convenient to find the optimum voltage with one LED laid on the water surface in the forward direction; it can be later changed so that the shine is optimum.
- You can rotate the LEDs and observe how the light intensity depends on the orientation of their terminals