Dependence of Boiling Point of Water on Pressure
Experiment number : 1707
Goal of experiment
This experiment shows that water boils at a temperature lower than 100 °C at a pressure lower than atmospheric pressure.
Theory
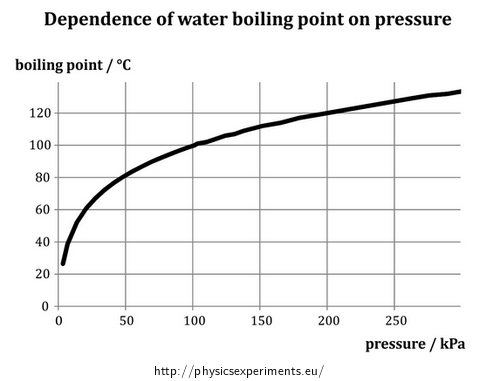
Evaporation of liquid in its entire volume is known as boiling. The boiling point is generally dependent on the ambient air pressure – the graph in Figure 1 shows this dependence for water. Data for the graph was taken from the web site The Engineering ToolBox.
Thus with decreasing pressure the boiling point decreases and vice versa; it is clear that the dependence is not linear. In a narrow range of pressures, however, a linear approximation is used.
\[\{t_b\}\,=\,71.6\,+\,\frac{7}{25}\{p\}\]wheretb is the boiling point in degrees Celsius and p is the pressure in kilopascals. In our experiment, however, we focus only on a qualitative investigation.
Tools
Procedure
First part:
In the first part of the experiment, we show the pressure changes in a vacuum container if we pump out the air.
We turn on the barometer and insert it into the vacuum container so as we can read the display.
We begin to pump the air out and we monitor the barometer display – See Video 1. In doing so we have to be careful not to expose the instrument to a very low pressure, which could damage it!
Video 1
We now know that by pumping the air out of the vacuum container we take away the air and therefore the pressure decreases
Second part:
We boil water in the kettle.
After switching off the kettle we wait until the boiling stops and then pour the water into the vacuum container (to a half of its height).
We begin to pump and watch the behaviour of the water inside.
Sample result
Video 2 shows how water begins to boil during depressurization at a temperature significantly lower than 100 °C.
Video 2:
Technical notes
Vacuum containers are financially accessible and they can be easily obtained in kitchen utensil shops. This experiment requires a transparent container.
Before performing this experiment, it is necessary to determine the measuring range of the barometer in order to avoid any damage. In vacuum containers it is usually not a problem to reduce the pressure to less than 40 % of atmospheric pressure!
During the experiment, it is necessary to follow the safety rules when working with hot water.
Pedagogical notes
For students it is not obvious whether during the pumping the pressure inside the container increases or decreases (although the name vacuum container suggests it). Therefore, it is appropriate to carry out both parts of the experiment, as described above; of course, if there is not an adequate barometer, we perform only the second part. But be sure to comment it thoroughly.
Alternative arrangement of the experiment
The experiment can be demonstrated in a more simple arrangement using only a syringe. We fill it with water from the kettle to approximately a half its volume; then we pull the plunger as far as the syringe construction allows. We can see the water boiling similarly as in the Video 3. The advantage of this arrangement is that it minimizes the necessary tools; the disadvantage is its small dimensions for a frontal performance.
Video 3:
Link to a related task
A graph of the boiling point of water with respect to pressure in the Theory part does not consider the pressure higher than 300 kPa. However, it is possible to determine mathematically the values of boiling point at higher pressures – such as in the task Boiling Point of Water At High Pressure. In this task, boiling point at 400 kPa is calculated.