Critical State of Carbon Dioxide
Experiment number : 1771
Goal of experiment
The goal of this experiment is to demonstrate how liquid carbon dioxide and its saturated vapour pass to supercritical state during heating and back to subcritical state during cooling.
Warning
It is unlikely that you would perform this experiment “live” during a lesson. Its implementation is tied to a unique tool. But we want to show the students the critical state at least in the form of the attached video.
Theory: Phase diagrams
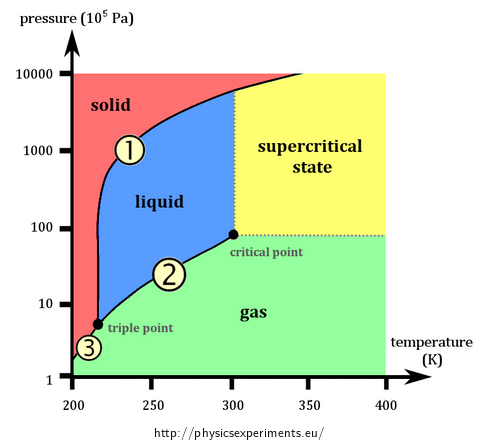
Almost every chemically pure substance may exist in solid, liquid or gas state. The state of the substance (of constant mass) is decided by its temperature and pressure. Defining state by these two quantities is usually visualized in a so-called phase diagram – example of such a diagram for carbon dioxide is shown in Fig. 1. The phase diagram is formed by three curves:
Melting curve (marked by number 1) connects the states, in which substance in solid state is in equilibrium with its liquid state; it therefore separates the liquid and solid state.
Saturated vapour curve (2) connects the states, in which liquid is in equilibrium with saturated vapour; it separates the liquid and gas state.
Sublimation curve (3) connects the states, in which substance in the solid state is in equilibrium with its saturated vapour; it therefore separates the solid and gas state.
The equality of chemical potentials of the two phases applies at each curve. The curves meet at a so-called triple point, where the substance coexists in all three phases in equilibrium.
Fig. 1 was taken from Wikipedia.
Note: The phase diagram may be generally multidimensional; resp. there may appear other than state variables pressure and temperature on the axes in two dimensions (typically thermodynamic potentials).
Theory: Critical state
As can be seen in the picture, the saturated vapour curve ends in a so-called critical point, which is characterized by critical pressure pc and critical temperature Tc. Table 1 shows these values for some selected substances; except for pressure and temperature critical density ρc is also listed.
Tabulka 1: Critical state variables of selected substances
Substance pc (MPa) Tc (K) ρc (kg/m3) water 22.1 647.1 322 ethanol 6.1 513.9 276 carbon dioxide 7.4 304.1 469 ethan 4.9 305.3 203 nitrogen 3.4 126.2 313 helium 0.2 5.2 70 At a critical point (critical state) the boundary between liquid and gas state vanishes, there are no differences in density, surface tension disappears and the specific latent heat of vaporization is zero – see the task Evaporation of Water and Ethanol (with Thermal Imaging Camera), More about specific heat of vaporization.
At temperatures and pressures greater than critical value pc and Tc the substance is in a state that is neither liquid nor gas, usually called a supercritical fluid (Fig. 1).
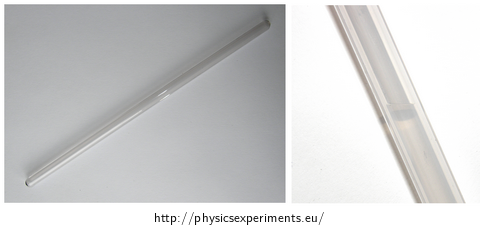
Tools
Procedure
Fill the beaker with water with temperature of about 45 °C to 50 °C which will serve as a water bath for the rod with carbon dioxide.
Turn on the slide projector and place the rod in front of its lamp, so that the liquid level of carbon dioxide shows on the screen.
Insert the lower end of the rod into the water and warm the rod until the boundary between liquid and gas state vanishes.
Once the boundary vanishes, remove the rod from the water and let it cool down, until the original state restores.
Sample result
Successful performance of the experiment is shown in the video below. Liquid carbon dioxide is at the beginning of the experiment in the lower part of the rod, the saturated vapour is above it.
The first part of the video shows the transition of the liquid carbon dioxide and its saturated vapour over the critical state (when the boundary between liquid and gas vanishes) to the supercritical fluid.
The second part of the video follows the opposite process, i.e. the transition of supercritical carbon dioxide through a critical state back to the initial state arrangement.
Technical notes
WARNING! Because carbon dioxide in the glass rod is stored at a pressure higher than 7 MPa, it is necessary to carry out the experiment with extreme caution and avoid any shock, let alone the risk of falling, etc.
Pedagogical notes
In the video, it is possible to register a distinctive darkening of the inside of the rod when the carbon dioxide passes from supercritical state to “normal”; state. It is good to inform the students that this is caused by the projection of the rod; in reality, white fog is created inside the rod, which obstructs the passing light.
Students can read from the graph that gas at supercritical temperature cannot be liquidized by compression (no matter how big); however, it can lead to its desublimation.
We can also tell the students that the transition to the critical state in not observed only at the boundary between liquid and gas, but also on the boundary between two immiscible liquids.
Critical state can be described also mathematically as an inflection point on a so-called critical isotherm in a pV-diagram, i.e. with condition:
\[\left(\frac{\partial p}{\partial V}\right)_T\,=\,\left(\frac{\partial^2 p}{\partial V^2}\right)_T\,=\,0\]If we substitute into these conditions the dependence \(p\,=\,p(V)\) for real (van der Waals) gas, we can get an estimation of the critical temperature and pressure for a given substance. Unfortunately, the van der Waals model is not designed to describe the substance in the vicinity of critical state; therefore the obtained results are very inaccurate.
Another video
Another high-quality video sequence showing the critical state of carbon dioxide can be found e.g. here.
More about supercritical fluids
It may be interesting for the students to note that supercritical fluids can be for example found as the atmosphere of some planets. The atmosphere of Venus is composed mainly of carbon dioxide (96.5 %) and nitrogen (3.5 %). Because the atmospheric pressure at the surface of Venus is higher than 9 MPa, and the mean temperature is over 700 K, it is clear (after consulting Table 1) that both of these substances exist as supercritical fluids.