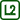Melting Point of Sodium Thiosulfate Pentahydrate
Experiment number : 1766
Goal of experiment
The goal of this experiment is to measure the temperature of sodium thiosulfate pentahydrate during heating and estimate the melting point of the substance.
Theory
The temperature at which a crystalline substance changes its state to liquid is called a melting point tm. This is one of the basic physical characteristics of crystalline substances – amorphous substances change to liquid state by gradually decreasing their viscosity.
When the crystalline substance is being heated, its temperature stops increasing after reaching the melting point; all supplied heat is used to break the structure of the crystalline lattice. When all of the substance changes to liquid, its temperature begins rising again thanks to supplying heat. Such temperature behaviour is indicated in the graph in Fig. 1.
The melting point generally depends also on the pressure and is affected by additives – e.g. table salt added to a mixture of ice and water extracts the heat required for its dissolution from the mixture and significantly decreases the melting point of ice.
Tools
Sodium thiosulfate pentahydrate, glass container or tube, laboratory stand with brackets, temperature sensor cooperating with a computer (in the model experiment the Vernier Go!Temp sensor was used), burner or candle, matches.
Sodium thiosulfate pentahydrate is a white crystalline substance with a melting point between 48 °C and 49 °C, which makes it ideal for this experiment. The substance is not subject to any P-statements or H-statements.
Procedure
Pour about 20 grams of sodium thiosulfate pentahydrate in a glass container (tube).
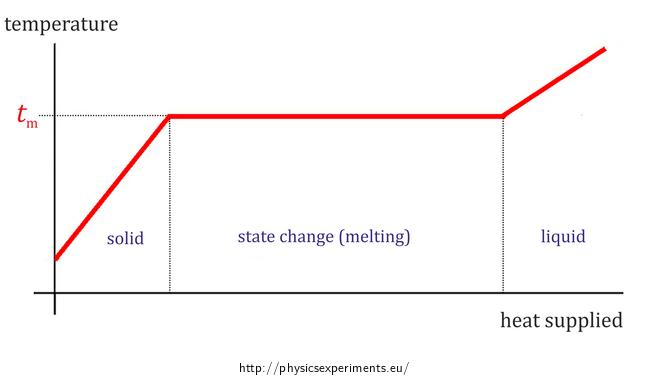
Fix the container in the laboratory stand, so that the bottom of the container is about 15 – 20 cm far from the burner.
Connect the temperature sensor to your computer and insert it into the container, so that its measuring element (its tip) stays immersed in the substance even after melting. This arrangement is shown in Figure 2.
Launch the programme for data collection from the temperature sensor and set the measurement time for at least 25 minutes.
Start the data collection and at the same time ignite the burner and set it to a very small flame.
Stop the measurement after the temperature of the sodium thiosulfate pentahydrate exceeds 60 °C.
Vzorový výsledek
After the measurement, we obtain the following graph of temperature versus time (Fig. 3).
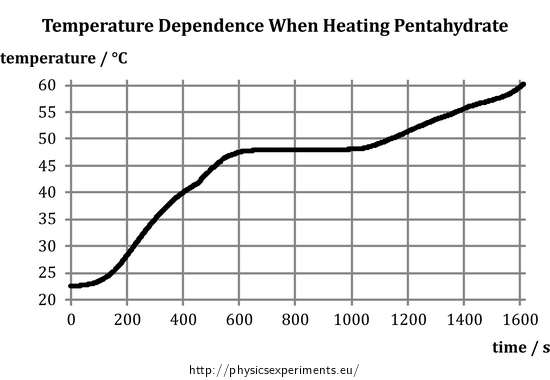
It is evident that in the first part of the measurement the temperature increase is approximately linear, then the temperature stabilizes and at the end of the measurement, it begins to increase again. To determine the melting point of the pentahydrate we focus on the middle part of the graph, when the pentahydrate temperature, despite continuing supply of heat, remains constant – the substance is melting. The melting point tm can be estimated from the y axis as approximately 48 °C.
Technical notes
Do not change the intensity of the flame during heating.
If you want to accelerate the measurement, it is possible to intensify the heating of the pentahydrate, or you can place the container nearer to the flame. However, there is a risk that the temperature stabilization will not be as distinctive, or the middle part of the graph will be much shorter.
If you use an alcohol burner or a propan-butan burner, make sure that there is enough fuel for 25 minutes burning before starting the measurement.
After the measurement, the melted sodium thiosulfate pentahydrate can be disposed by pouring it into the drain.
Pedagogical notes
If we consider the parts of the graph in which the temperature increases to be linear, we can observe that the gradients of these dependencies are different – the temperature of liquid pentahydrate rises more slowly than of solid pentahydrate. The container, however, is heated by the same heat input. Therefore, we can assume that the specific heat capacity of liquid pentahydrate is grater that the specific heat capacity of solid pentahydrate (similar effect can be observed with water and ice).
This experiment typically takes a relatively large part of a lesson (e.g. one half). However, during this experiment, you can attend to other activities – instruction, examination etc. It is not necessary to monitor this experiment.
Do not touch the container or the stand right after the measurement. There is a danger of burning!