Thermal Effects of a Laser Beam
Experiment number : 4348
Goal of experiment
The goal of the experiment is to show that the visible component of electromagnetic radiation also carries energy, the absorption of which causes temperature increase of objects.
Theory
When we talk about heat exchange by radiation in the school environment, we are very often talking about infrared radiation – we come across the claim that we as humans radiate the most at wavelengths around 10 µm, as well as objects of room temperature around us. The fact that the wavelength range of 8 µm to 15 µm is sometimes directly referred to as thermal radiation (see e.g. English Wikipedia), may then lead to the impression that if a body is heated up by radiation, it must necessarily be the cause of infrared radiation of these wavelengths – the reality is somewhat different. For any body, which we will consider as a black body for simplicity (more about black body can be found, for example, here), the so-called Wien’s displacement law applies in the form:
\[\lambda_\mathrm{max}T\,=\,b\tag{1}\]where the constant \(b\) is \(b\,=\,2.9{\cdot}10^{-3}\,\mathrm{mK}\) and \(\lambda_\mathrm{max}\) is the wavelength at which the black body with surface temperature T irradiates the most. According to this law (which is based on the more general Planck's law), bodies at room temperature actually radiate most at wavelengths around 10 µm, but for example the Sun, with a surface temperature of almost 5800 K, has a maximum of its radiation shifted to about 500 nm; much of its energy is therefore radiated in the visible region as light. The sun therefore warms us not only with its infrared radiation but also with its visible radiation.
This experiment uses a laser diode (which we know more commonly as a laser pointer), with radiation of a completely different nature than the above radiation emitted by people or the Sun. The radiation in laser diodes is due to electron jumps in PN transitions of the semiconductors used and is emitted over a very narrow range of wavelengths; the “Planck” radiation of the diode related to its temperature according to relation (1) is, by contrast, quite negligible. The laser we use emits radiation at a wavelength of 532 nm, which corresponds to the colour green; in the experiment we will show that even this visible radiation has significant thermal effects.
Equipment
Thermal imaging camera, black styrofoam board, laser pointer with a holder to fix it in a horizontal position.
The laser pointer used has a rated power of up to 5 mW and operates at a wavelength of 532 nm (green); its construction and features are described in more detail in Thermal Effects of a Laser Beam, Design of green laser pointer. The black polystyrene plate used in the sample is used as a serving tray for packaged sliced cheese.
Procedure
Turn on the thermal imaging camera and set it to maximum mode, which is able to find and measure the temperature of the hottest spot in the selected white-framed square.
Fix the laser pointer against the black plate so that the trace is always pointing to one place (see Figure 1). It is useful to also arrange for the pointer to be on continuously (for example, by taping duct tape over the on button).
Using a thermal imaging camera, monitor the illuminated area. Within a few seconds to tens of seconds, the temperature can rise locally up to 100 °C.
Sample result
A successful execution of the experiment is illustrated in real time in the video below.
In this video, the FLIR i7 thermal imaging camera was used. The temperature range of the colour scheme was set in the interval of 25 °C and 31 °C, the emissivity was ε = 0.95.
Technical notes
Of course, you can also use a plate made of any insulating material other than polystyrene; in general, plastic objects with a black coloured matt surface can be recommended.
It should be taken into account that due to high temperature the plastic may locally deform – melt, bend, etc.
We do not recommend using this experiment for any quantitative measurements – the laser power fluctuates over time and decreases with the gradual discharge of the batteries.
Safety notice: Follow the principles of laser radiation safety when working with the laser pointer!
Pedagogical notes
The idea that a laser beam has measurable thermal effects is inherent for the students – partly due to the influence of science fiction movies and books. But when we try shining the same laser pointer we used in the experiment on, say, our hand, we don’t feel any temperature increase – and this despite the fact that the plastic was heated to 100 °C. A natural question for students, then, is how they would explain this discrepancy. The solution lies in the different thermal conductivities of plastic and skin: while in the case of plastic, which is an excellent insulator, all incident energy is concentrated in a very small area, human skin distributes the delivered heat over its surface and therefore no significant local heating occurs. To demonstrate this fact, we can replace the plastic with a matte black metal plate for a moment – no local heating is observed here either.
If we have a spectrometer at our disposal, we can directly convince students that the green laser pointer emits dominantly light of one wavelength, i.e. that this part of the spectrum is responsible for the thermal emission. The spectrum of the laser used is shown in Figure 2.
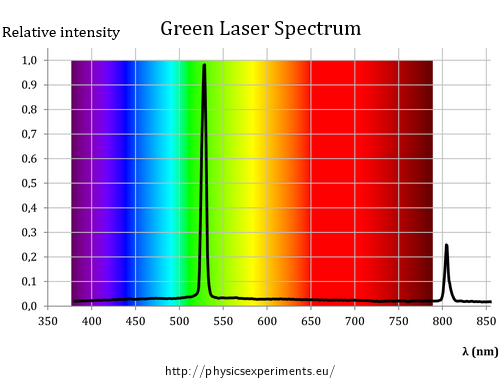
In addition to the aforementioned 532 nm, there is one spectral line in the near-infrared region, more precisely corresponding to a wavelength of 808 nm; its origin is explained in section Thermal Effects of a Laser Beam, Design of green laser pointer.
Design of green laser pointer
The production of a red laser pointer is technically a relatively simple matter, because it is not a problem to directly construct a diode with the desired wavelength. However, the construction of a green laser (with typical wavelength 532 nm) is more demanding. Green light is obtained by an indirect process that begins with an infrared laser diode made of aluminum gallium arsenide emitting at a wavelength of 808 nm. Using the crystal Nd:YVO4, this radiation is converted to a farther infrared length of 1064 nm, and by doubling the frequency of this radiation, the resulting green light is received at a wavelength of 532 nm.
Since the wavelength of 808 nm may not be completely filtered from the resulting laser beam, we can find it in the spectrum of the green laser as seen in Fig 2.
The technology of the whole process is described in more detail, for example, at Wikipedia.
Thermal imaging camera basics – link to PDF
In this experiment, a thermographic measurement is used. The theory of thermography and basic recommendation and procedures that can help you obtain more accurate and undistorted results can be found in Experiments with thermal imaging camera (in Czech only).
Alternative experiment without thermal imaging camera
This experiment can also be performed without a thermal imaging camera, using a point thermometer; in the sample experiment, a Vernier STS-BTA point temperature sensor was used. We shine a laser beam on the measuring element of the sensor (Figure 3) and observe the increase of the measured temperature.