Heat Dissipation Through a Copper Plate or How to Speed up Cooling of Tea
Experiment number : 1945
Goal of experiment
We will measure how much the cooling of hot water in a container will change when we put a copper plate under the container.
Theory
If we put a container filled with hot water on a table, a few mechanisms of heat exchange will work simultaneously during its cooling. First of all, the liquid evaporates, therefore loses the particles with the highest kinetic energy and its overall temperature decreases; part of its energy is also released into its surroundings as infrared radiation; and part is used to heat the surrounding air and the desk under the container due to heat dissipation.
If we put a copper plate underneath the container, we can influence the last factor mentioned, that is heat dissipation. Copper is a very good thermal conductor and heats very easily in its whole volume when in contact with the container – the container therefore transfers energy to the plate and cools faster.
Tools
Copper plate, two identical containers, kettle, two temperature sensors (ideally connected to a computer), eventual stative material to conduct the experiment.
Two stainless steel temperature sensors Vernier and a copper plate with dimensions 400×150×0 mm were used in the sample experiment.
Procedure
We put the containers next to each other on a table and put the copper plate under one of them.
We fasten the temperature sensors to the same height so that each reaches into one container
We set the measurement time to at least 15 minutes.
We boil water in the kettle and pour it into both containers to the same height; the temperature sensitive part of the sensors (usually the tip) must be submerged. We start the measurement of temperature simultaneously and observe its development.
The layout of the experiment is depicted in Fig. 1.
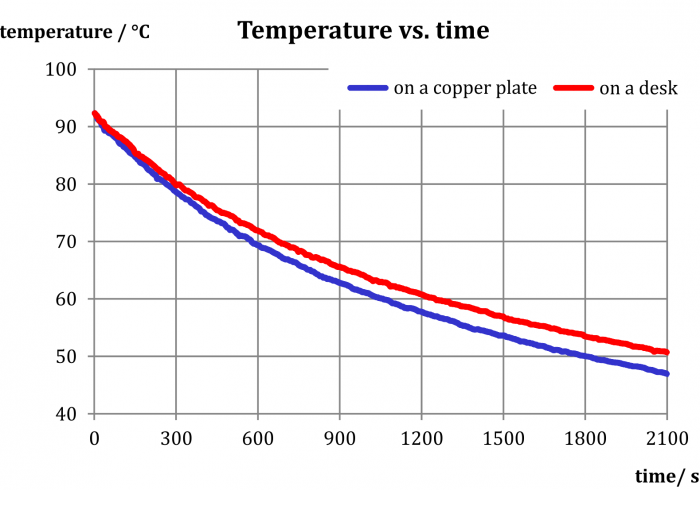
Sample result
Technical notes
We of course do not stir the water in the containers during our measurement; otherwise the results could be significantly altered.
The measured effect disappears when the desk is made out of a highly conductive material – the role of the copper plate is then practically fulfilled by the whole desk for both containers and the differences become negligible.
Pedagogical notes
As the experiment shows, the effect of a copper plate on the cooling of water is small – the difference in temperatures is only 3 °C after more than half an hour. It seems that in this case heat dissipation is not the main mechanism through which water cools down.
We can challenge the students to think of ways to increase the difference in temperatures (therefore the gap between the curves). The natural options include replacing copper with an even better heat conductor (though there are not many) or insulating the second container from the desk, for example with a polystyrene pad.