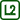Ice Cubes Cutting
Experiment number : 1943
Goal of experiment
The goal of this experiment is to demonstrate the different thermal conductivity of two metals, copper and brass.
Theory
See the theory of a previously described experiment: Thermal Conductivity of Plastic and Metal I., Theory.
Tools
Two identical ice cubes, copper plate and brass plate of the same size (0.3 mm thick in this experiment), insulating pad (wooden block, polystyrene), lab stands if necessary.
Procedure
We place an ice cube on a table or on an insulating pad (a wooden block in our case).
We place one of the plates on it so that it presses perpendicularly on the cube with its shorter edge (Fig. 1). The plate in Fig. 1 is not fixed in the holders, it merely rests upon them to stay in a vertical position.
We let the cube be cut by the weight of the plate.
We repeat the experiment with a new cube and the second plate. We observe the speed with which the plates traverse through the ice.
Sample result
A successful execution is illustrated in the video below (time is sped up twice). We can see very clearly that the copper plate penetrates ice much faster than the brass plate. The heat conductivity of copper is about three times higher than that of brass. That means the copper plate is able to transport much more heat to its colder areas, which in turn speeds up the melting of ice.
Technical notes
We can of course carry out the experiment without the stands by taking the plate into our hand and pushing it down, cutting the cube. This approach makes it difficult to ensure the acting force is constant (and therefore comparable for both plates).
Ice melts during the experiment. It is appropriate to work on a pad that cannot be damaged by water.
Pedagogical notes
To be more illustrative, it is ideal to have two sets of stands and two insulating pads so that we can conduct the experiment in parallel with both copper and brass (as is depicted in the video above).
We can of course use other materials than copper and brass – aluminium, iron etc., but it is important to make sure both plates have approximately the same mass. Otherwise students can reach the natural conclusion that the heavier plate will cut through the ice faster.
If we choose brass and copper, the materials from the sample experiment, we only need to make sure the plates are of similar size – density of brass and copper is almost identical.
Students can know the fact that the melting point of ice locally increases with higher pressure caused by weighing down the cube (for example in relation to the so called regelation of ice). However, if we use plates with the same mass and the same thickness of the cutting edge, this fact can in no way impact our experiment.
Another way to show the negligible effect of regelation is a direct calculation of the pressure caused by the plate. It is about 150 kPa in the sample experiment, which is an increase in pressure that would translate to a decrease in the melting point of ice of about 10 mK.
We can verify whether the students understood the experiment with a follow-up question: “How would the experiment end, if the copper plate were for example twenty times smaller than the brass one (maybe just a bit bigger than the cross section of the ice cube)”. Students should then, with the teacher’s help, come to the conclusion that in such a scenario the whole copper plate would quickly cool down to a temperature close to 0 °C. Subsequent melting would then be very slow and the cube would probably never be fully cut. (It is reasonable to support this hypothesis with an experiment.) This also shows that the request for similar sizes and masses of both plates is justified.