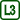Height of Liquid Level in Capillary Tube
Experiment number : 2123
Goal of experiment
The goal of this experiment is to show the dependence of height of the water level in the capillary vs. the capillary diameter and water temperature.
Theory – Capillary action
If a capillary (a tube with very small internal radius) is immersed into a liquid, the liquid level in the capillary is above (or below) the liquid level outside the capillary.
When a liquid wets the container walls, it causes capillary elevation – liquid level in the capillary is higher than the liquid level outside the capillary.
Non-wetting liquids cause capillary depression – the liquid level drops below the level outside the capillary.
The distance h between the levels of a liquid inside and outside the capillary can be determined from relationship
\[h = \frac{2σ}{rρg},\tag{1}\]where σ značí povrchové napsignifies the surface tension of the liquid at given temperature, r is the capillary radius, ρ is the liquid density and g is the gravitational acceleration.
Note: Derivation of this relationship can be found in the task Capillary action between glass plates.
From the relation (1) we can see that with increasing radius of the capillary, the height of the liquid level in the capillary decreases.
This experiment is better performed with coloured water. The height of the level will, in addition to the radius of the capillary, also depend on water temperature. Surface tension of water decreases with increasing temperature. Water density also decreases with increasing temperature (from about 4 °C)
Note: To solve a similar problem, see Height of Water in a Capillary Tube.
Tools
two or more capillaries of different radii
capillary holder
water container
kettle
water
two different food colourings (not essential, however the result is better seen when the water is dyed)

Procedure
Dye cold water with food colouring.
Immerse a capillary into the water.
See how the high of the water level in the capillary rises.
You can mark the height of the water level in the individual capillaries with a marker or an adhesive paper.
Repeat with hot water.
Sample result
For our experiment, we used cold water at a temperature of approximately 10 °C, which we dyed with a blue food colouring, and hot water at about 70 °C dyed red.
Figure 2 shows capillary elevation in the case of cold water (left) and hot water (right).
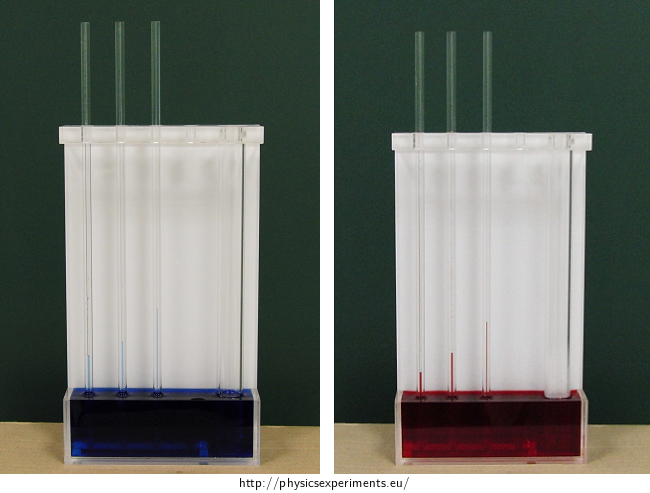
Figure 3 shows a detail of the capillaries with cold and hot water. To compare the height of the levels we lead lines from capillaries in cold water to the capillaries with hot water. You can see that cold water rises higher than hot water.
Pedagogical notes
The height reached by the water level in the capillary can be measured and compared with the calculated height according to relationship (1). You can discuss with the students whether the measured value corresponds to the theoretically calculated height of the capillary elevation, or why these values differ.