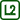Comparing Thermal Conductivity of Copper, Aluminium and Brass
Experiment number : 1769
Goal of experiment
The goal of this experiment is to use thermosensitive films to visualize the different dynamics of heat conduction in three different metals.
Theory
See theory in an already described experiment: Thermal Conductivity of Plastic and Metal I., Theory.
Tools
Thermosensitive film with temperature range from 25 °C to 30 °C, three different metal plates of the same size, container for hot water, kettle.
In a sample experiment, copper, aluminium and brass plates of the same dimensions are used; the thickness of the plates is 0.3 mm. (Similar metal plates can be obtained in designer tools store). A table with thermal conductivities (at 25 °C) of used metals is stated below:
metal λ / W·m−1·K−1 copper 386 aluminium 237 brass 120 Thermosensitive film can be found on the internet under the name reversible temperature label. Figure 1 shows a tool made especially for this experiment to study the different thermal conductivity of metal – three different metal plates are partly covered by a thermosensitive film, which indicates the temperature increase.
Procedure
Fasten the copper, aluminium and brass sheet parallel to each other (see for example Fig. 1) using a laboratory stand so that the ends of the sheets are several centimetres above the table (Fig. 2). Put a container under these ends and pour hot water into it so as to cover the ends of the sheets.

Observe the thermosensitive films change colour. The temperature represented by the colour depends on the film type. The film used in this experiment is black at temperatures lower than 25 °C. If the temperature increases, in the interval from 25 °C to 30 °C the film gradually changes its colour from brown, green and blue to navy blue, and finally after exceeding 30 °C the colour changes back to black.
The purpose of such colour changing of these films is not to attempt precise temperature measurements at a particular point, but rather to indicate and demonstrate the distribution of surface temperature.
Sample result
A successfully performed experiment is illustrated by the video below. The video is speeded 8 times.
It is apparent that the copper sheet heats the fastest, followed by aluminium and brass.
Technical notes
Do not pour boiling water in the container, use water with temperature of 60 °C. At higher temperatures, a large amount of hot vapour is generated and it flows upward affecting the measurement with thermosensitive films and making it unreliable.
The effect stated above can be eliminated by bending the bottom ends of the sheets to right angle. Thus the longer part of the measured metals can stay in a horizontal position.
If you perform this experiment in the summer, it is recommended to make sure that the temperature in the classroom is lower than the minimum temperature measured by the film (here 25 °C). If the classroom temperature is higher, the film changes colour to the corresponding temperature making the result less evident.
It is not necessary to use hot water to heat the sheets. However, you always need to ensure that the sheets warm up evenly.
Pedagogical notes
The description of the development of this experiment leads the students to a conclusion that “copper heats faster than aluminium” etc. More proficient students can figure out that we have already discussed the “willingness” of matter to change temperature in the context of specific heat capacity c of matter. This thought is correct and should be appreciated – the willingness of matter to change its temperature depends on the specific heat capacity as well as the thermal conductivity of matter.
The argument that fast heating of the copper sheet is caused by its low heat capacity can be easily confuted with the table below:
metal λ / W·m−1·K−1 c / J·kg−1·K−1 copper 386 383 aluminium 237 896 brass 120 384 Therefore, if the decisive factor were specific heat capacity of the metal, the behaviour of copper and brass would be almost the same (they have similar values of c), but this is in clear contradiction with the experiment.
If there are truly gifted physicists in the class, they can object that this argument is not entirely satisfactory – the sheets have different density and hence the mass, which affects the size of the heat needed for the heating, is also different. Fortunately, the densities of copper and brass are similar enough so that different behaviour of these two substances cannot be explained in other way than on the basis of different thermal conductivity.
The effect of different thermal conductivity can be demonstrated not only with heating the metals, but also with cooling them. Let all three metals warm up, for example on a radiator, until the thermosensitive films are navy blue. Then submerge the ends of the metal sheets in a mixture of water and ice. Copper cools down the fastest, followed by aluminium and brass.
In the interpretation of the extension of the experiment, we should be careful not to give the impression that the sheets “sucked” the cold out of the ice – it is always necessary to interpret the temperature decrease as a heat extraction.
Alternative performance of the experiment
To demonstrate the difference in thermal conductivity of the three metals, you can proceed differently. Lay the copper, aluminium and brass sheet on the table and in the middle of each sheet place an ice cube. Watch how quickly individual cubes melt (the video is speeded 32 times):
It is apparent that ice melts the fastest on a copper sheet, and the slowest on the brass sheet. Copper has high thermal conductivity and therefore is able to constantly supply heat from the peripheral parts of the sheet to the place cooled by the ice cube. This ability is significantly worse in the brass sheet.
The advantage of this experiment is the possibility to go without the thermosensitive films. The disadvantage is that it is more time demanding (approximately 15 minutes).