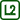When Carbon Dioxide is Lighter Than Air (Temperature Impact on Gas Density)
Experiment number : 2258
Goal of Experiment
The goal of this experiment is to show how temperature of a gas affects its density.
Theory
We have three lit candles of different heights, that are covered by a container. The candles go out after a short time. Burning of candles produces carbon dioxide, which prevents burning.
At room temperature, carbon dioxide has higher density (\(\rho\) = 1.98 kg·m-3) than air (\(\rho\) = 1.29 kg·m-3). The density of gas, however, significantly depends on its temperature. Carbon dioxide produced by burning has higher temperature than the surrounding air. The density of carbon dioxide is therefore lower than the density of cold air around the candles, so carbon dioxide rises upwards and accumulates at the top of the container. This is the reason why the longest candle goes out first and the shortest last.
Equipment
- Three candles with different heights
- Play dough
- Fire-proof container (glass)
- Matches
Procedure
- Arrange the candles according to their height.
- Use the play dough to create a holder for the candles.
- Light the candles and let them burn for a while.
- Cover the burning candles with a glass container.
Sample Result
The video below illustrates a successfully implemented experiment.
Technical Notes
If the container is too big, the experiment will take a long time.
The bigger the difference in height of the candles, the more explicit the result.
If the candles are too close to the walls of the container, they can blacken them.
Pedagogical Notes
Before conducting the experiment, it is advisable to ask the students what result should be expected. Usually, the students incorrectly predict that the short candle will go out first, since they use the assumption that under normal conditions, carbon dioxide has higher density than air. If we want them to consider the impact of temperature on the density, we can prompt them with a question: “Is it possible, that sometimes carbon dioxide is lighter than air?” The following discussion should lead to the fact that the density of gas depends on its temperature.
Mixing of hot carbon dioxide and cold air in the container is very complicated; the circulation of gases constitutes a process that is difficult to describe. This is one of the reasons why the candles go out at a different rate each time we conduct the experiment, even though the layout of the experiment is still the same.
A More Detailed Experiment
As another experiment, we can show that the candle goes out even though it is not separated from the surrounding air. In addition to the above mentioned equipment, we will need a tripod stand:
- Arrange the experiment according Figure 2.
- Light the candle and cover it by the container.
- Observe the change of the flame and wait until it goes out.
A sample experiment is shown in the video below. The process lasts a few minutes, depending on the size of the container, its placement and the flame of the candle; the whole flame must be above the lower brim of the container. The fact that the flame goes out indicates that even though air can get in the container from below, a sufficient amount of carbon dioxide accumulates in the upper part of the container so that it prevents burning.
We can ask the students how would they arrange the experiment in such a way, that the candles would not go out. A simple solution is to cover the candle with a container with a hole at the top. This gets us to the principle of an oil lamp: its flame is also covered by a glass container, but hot residues can get out through the hole at the top. There is also a chimney effect that brings fresh air to the flame.