Water droplets on various surfaces
Experiment number : 4302
Goal of experiment
The experiment shows the relationship between the shape of a water droplet and the surface on which it lies.
Theory
If water is poured into a container, different curvatures of the surface can be observed at the surface edge. The shape of the surface depends on the resulting forces between the molecules of the liquid itself and between the molecules of the liquid and the surface. We are talking about the liquid ‘wetting’ or ‘not wetting’ the wall of the container.
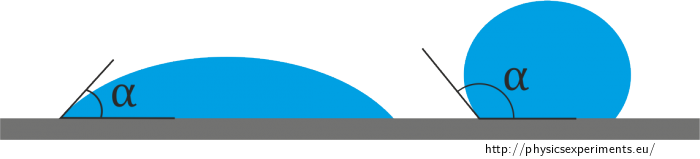
Similarly, if we drop a liquid onto a surface, the resulting droplet will have a different shape depending on whether or not the liquid wets the surface (see Figure 1). If the angle that the droplet makes with the surface (usually referred to as the contact angle) is less than 90°, the liquid is said to wet the surface. If, on the other hand, the angle is greater than 90°, the liquid does not wet the surface.
Whether or not a liquid wets a surface depends on the liquid itself, but also on the surface. In addition, both can be adjusted so that the wettability suits the need. Thus, for example, in practice, water with a detergent wets a Teflon pan better than water alone, a greasy porcelain plate is less wetted by water than a clean one, etc.
Note: Common surface treatments to make water less wettable include impregnation. However, some impregnations do not change the contact angle itself, but rather modify the surface so that the droplet does not soak in so quickly – so the shape of the droplet may hardly differ after the application of these impregnations.
Tools
- a container of water
- a dropper or plastic pipette
- food colouring
- various (preferably non-absorbent) materials (e.g. glass, acrylic glass, wax, Teflon, paper, polystyrene, fabric, etc.)
- impregnation, if necessary
Procedure
Gradually drip water on different surfaces and observe the shape of the drops. The surfaces can be divided according to whether or not the water wets them or sorted according to the estimated size of the contact angle, etc.
Sample result
Examples of droplets on some surfaces are shown in Figure 2. It can be seen that e.g. paper or acrylic glass is significantly wetted by water (contact angle is much less than 90°), whereas fabric with impregnation or Teflon is not wetted by water.
Note: For acrylic glass, you can see a “kink” at the bottom of the droplet, which is caused by reflection on the shiny surface of the acrylic glass. The drop itself ends, of course, at the point where we see the refraction.
Pedagogical notes
- The experiment is designed to try to remove the common misconception that “water wets and mercury does not wet” – the aim is to show that wetting depends not only on the liquid but also on the surface. For this reason, only water is used here. However, the activity can of course be extended by observing drops of different liquids on the same surface, or comparing the behaviour of water and water with a detergent.
- The experiment can be done in groups as a part of laboratory work, or you can have photographs prepared in advance for the students to work with (sorting them according to how each droplet wets, estimating or measuring the size of the contact angle, estimating which surface is involved, etc.).
Technical notes
- Droplets should not be too large to avoid deformation. Therefore, it is advisable to drop only one drop at a time on a given spot (which, at the same time, ensures that all drops of a given liquid are formed from the same volume of water due to surface tension).
- If you want to measure the contact angle from a photograph, you should always photograph the drop from the “front” (i.e. take a picture of the outline).
- For better visibility of the drops in the photographs, the water can be coloured with food colouring (especially suitable for light surfaces, whereas on dark surfaces the uncoloured drop is more visible).
- The effect of different impregnation types on the size of the contact angle can also be compared in the activity. In this case, it is necessary to prepare the impregnation samples in advance.