Cooling Mixture of Water, Ice and Salt
Experiment number : 2047
Goal of experiment
The goal of this experiment is to verify that adding kitchen salt into a mixture of water and ice can decrease the melting/freezing point of the mixture.
Theory
Melting points (or freezing points) of crystalline substances generally depend on the surrounding pressure and chemical pureness of the substance – possible impurities can considerably affect the melting/freezing point of the substance. Water can coexist with ice in an equilibrium state under normal pressure at a temperature of 0 °C. However, when we mix water, ice and salt (NaCl), this temperature decreases.
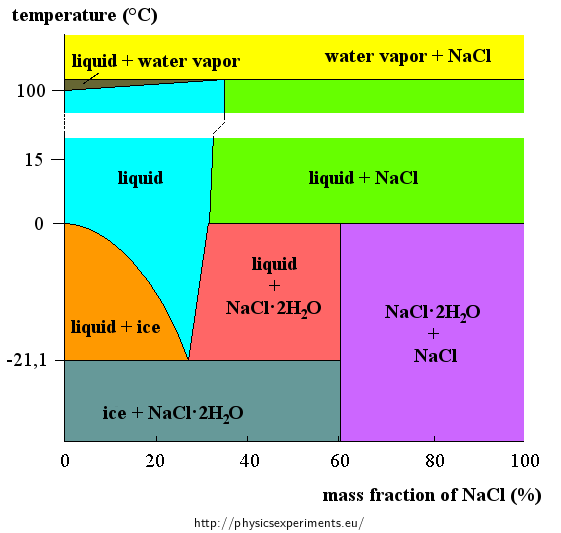
Figure 1 shows a phase diagram of a mixture of water and salt; the vertical axis shows temperature, the horizontal axis shows the concentration of salt. For each pair temperature-concentration can be found the state of the mixture.
Example: For 50% concentration of salt at temperature 15 °C the graph shows a green depicted phase “water+NaCl”, which means that some salt does not dissolve (supersaturated solution).
We can see from the graph that the lowest temperature, at which a mixture of water, ice and salt can exist, is ca. −21 °C and the salt concentration is approximately 23 %.
Therefore, if we add salt into a mixture of ice and water, the melting/freezing point of the mixture decreases and the ice begins to melt. In order for a phase change to occur, the ice draws the heat of fusion from its surroundings, which allows the temperature to decrease.
Tools
Ice, hammer, beaker, styrofoam block, water, kitchen salt, thermometer (Fig. 2).

Procedure
Use the hammer to crash some of the ice cubes and them into a beaker with a small amount of water. Insulate the beaker with a styrofoam block.
When the temperature of the mixture stabilizes at approximately 0 °C, start adding salt into the mixture while stirring it; use the thermometer to measure the temperature of the mixture.
Sample result
In the sample experiment we were able to reach the temperature of −15 °C without measuring the ratio between water, ice and salt.
Technical notes
When crushing the ice, it is advised to wrap the ice cubes in a towel or a rag (Fig. 3). Otherwise you will have pieces of crushed ice flying all around the room.
It is convenient to use such a thermometer that can be connected to a computer and plot the time dependence of the temperature.
A thermometer with a firm body can also be used as a stirrer.
The phenomenon is also observed when no insulation is used.
During the experiment, air humidity freezes on the surface of the beaker; the beaker can also freeze to the mat.
It is not necessary to use kitchen salt for this experiment. Same effect can be found when using other salts dissoluble in water (for example CaCl2, FeCl2, MgCl2 etc.).
Pedagogical notes
Practical use of this experiment is, apart from preparing a cooling mixture, also salting frozen roads in the winter. Practice shows that students do not know how the experiment is related to this practical use. (Suggestive question asked by the teacher can be: “I want the ice on the road to melt. How does it help that I decrease its temperature?”) Therefore it is necessary to emphasize, that adding salt primarily decreases the melting/freezing point of the mixture – therefore we can have liquid water even at temperatures well below 0 °C. In other words, from a frozen road we can make a wet road.
Attentive students can object, that intense stirring can be counterproductive, since mechanical work increases the internal energy of the mixture and the mixture therefore warms up. This reasoning is of course correct and it is good to appreciate it; on the other hand, it is advisable to point out that the temperature increase during stirring is in this case negligible.
Complementary experiment
A complementary experiment is to show that even dissolving salt in water can cause temperature decrease of the mixture. The physics behind this phenomenon is, however, different from the one used in the previous experiment – dissolving salt is an endothermic process, when the mixture draws heat from its surroundings to break the lattice of sodium chloride (we can talk about so called heat of dissolution), change in state does not occur.
Gradually pour some salt (at room temperature) into a glass with water at room temperature, stir the mixture and observe, that the temperature decreases. The video below illustrates the impementation of the experiment using the Vernier system.







