Latent Heat of Boiling (Qualitatively)
Experiment number : 2259
Goal of Experiment
The aim of the experiment is to prove the existence of latent heat of boiling.
Theory
If we supply heat to a liquid, its temperature will rise until the liquid reaches its boiling point. If we continue to supply it with heat, this heat will be used to convert the liquid into vapour. Only when all the liquid has been converted into vapour can the temperature of the substance increase. The heat that we had to supply to convert the liquid into vapour is called the latent heat of boiling.
The latent heat of boiling can be perceived as a special case of the so-called latent heat of vaporization. If we allowed all the liquid to evaporate at a different, lower, temperature (which would take much longer), the liquid would have to absorb more heat (latent heat of vaporization) for this transformation than if it changed state at its boiling point. More about the latent heat of vaporization and boiling can be found in the experiment Evaporation of Water and Ethanol (with Thermal Imaging Camera).
Equipment
- Thermocouple that can be connected to a computer
- Water container
- Candle
- Matches
Procedure
Light the candle and dip the thermocouple tip in water.
Let the water run down the body of the thermocouple so that a water drop forms on its tip (see Fig. 1).
Start the temperature measurement and put the thermocouple into the candle flame.
After the drop evaporates (it should take only a few seconds), stop the measurement.
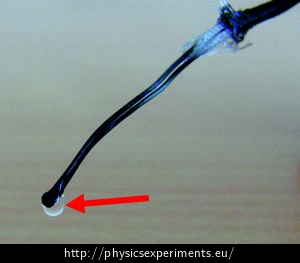
Sample Result
The Vernier TCA-BTA thermocouple was used in the sample measurement. You can use another sensor with a similar range that can plot a graph when connected to a computer. The graph below (Fig. 2) shows a sample of the measured data.
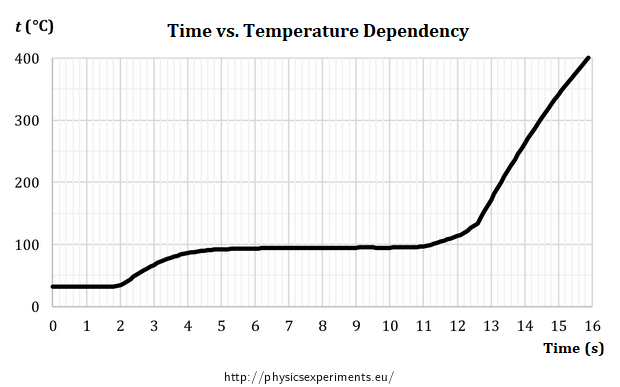
We inserted the thermocouple into the flame between 1st and 2nd second of the measurement. The temperature rises for about 6 seconds, when it reaches approximately 100 °C and the increase stops. The horizontal part of the graph in the range between 6 to 11 seconds shows that there is a latent heat of boiling, in this part the liquid changes to vapour in its entire volume and the temperature does not rise despite the supply of heat. Only after all the liquid water has evaporated (11 seconds) does the temperature of the thermocouple in the flame start to increase again.
Technical Notes
If the drop drips into the flame during the experiment, the experiment must be repeated.
The temperature does not have to stop at exactly 100 °C, as thermocouples usually operate over large temperature ranges and their accuracy can normally be in the order of ones of degrees Celsius.
Pedagogical Notes
In the discussion following the experiment, students can be asked what temperature is measured by the sensor after the drop completely evaporates. Together we should come to the conclusion that it is the temperature of the sensor itself and that this was the case not only after the evaporation of the drop, but throughout the whole measurement. Contact thermometers always measure the temperature of themselves, and we only assume that a thermodynamic equilibrium has stabilized between the thermometer and the measured substance, ensuring the same temperature of the bodies that are in contact.
If students already have some knowledge of phase transitions, we can ask them to sketch the temperature over time graph before the experiment .






