Supercooled Liquid
Experiment number : 2253
Goal of Experiment
This experiment aims at measuring the temperature vs. time dependence during solidification of liquid sodium thiosulphate pentahydrate to demonstrate the existence of a supercooled liquid.
Theory
A supercooled liquid is a liquid that exists in liquid state at a temperature lower than its freezing point (therefore this phenomenon cannot be observed at amorphous substances, since they do not have a definite freezing point). This state is metastable and in the event of disturbing stimuli (shocks, impurities), the likelihood that the substance spontaneously transitions to a more stable state, i.e. crystallizes into a solid state, increases. This transition then releases the latent heat that heats the substance to its freezing point.
For most liquids, supercooling is normally indistinct; however, for example water droplets in cumulus or cumulonimbus clouds do exist in liquid state even at temperatures of several tens of degrees Celsius below freezing point. In combination with cold earth surface, supercooled rain drops then create conditions for the formation of glaze in winter.
Equipment
Sodium thiosulphate pentahydrate, laboratory scales, laboratory stand with brackets, rod, burner, tripod with mesh, two different-sized beakers (approx. 50 ml and 200 ml), temperature sensor cooperating with computer (in the sample experiment, Vernier Go!Temp sensor was used).
Procedure
Connect the temperature sensor to the computer and set the measurement time to 30 minutes.
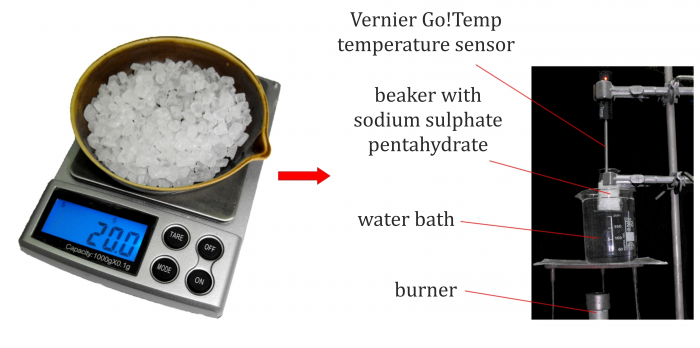
Measure about 20 g of thiosulphate and pour it into the smaller beaker. Fill the larger beaker with water creating a water bath.
Arrange the experiment as shown in Figure 1. A temperature sensor is inserted into the smaller beaker so that its measuring element (tip) remains submerged even after the pentahydrate has melted. The smaller beaker with pentahydrate is partially immersed in water bath.
Light the burner and start to warm the water bath. Continue until all solid pentahydrate has melted; then turn the burner off.
After turning the burner off, the thiosulphate is still being warmed by the water bath for some time; the temperature typically rises above 60 °C. Therefore, start the measurement when the temperature has dropped to 55 °C. Continue the measurement during the solidification process.
Sample Result
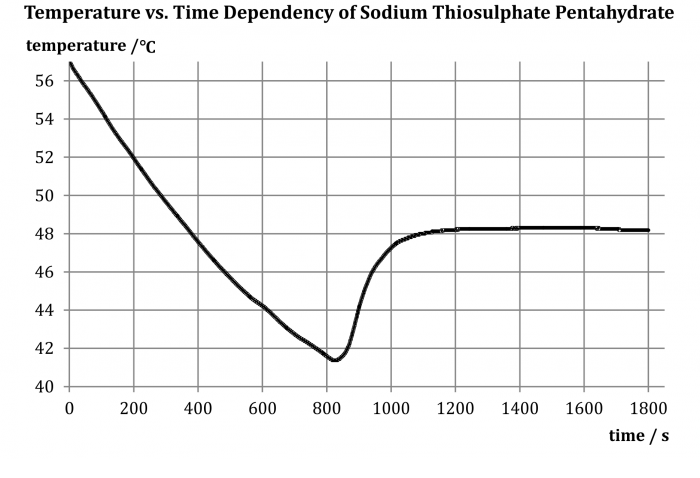
The measured dependency is shown in the graph in Fig. 2.
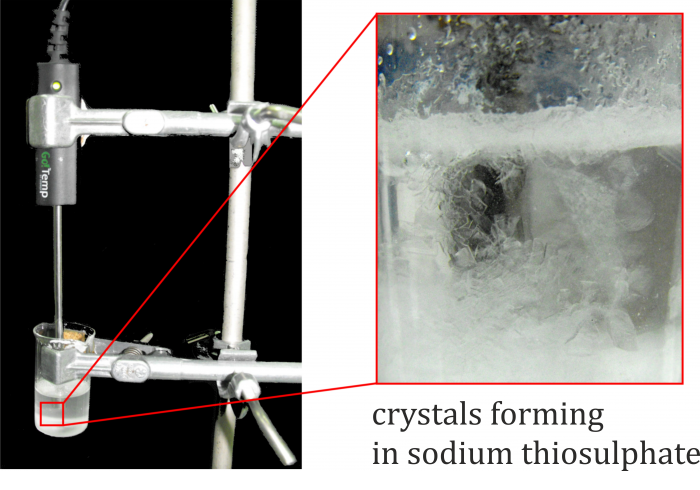
The commonly stated freezing point of pentahydrate is between 45 °C and 50 °C. From the measured curve it can be seen that its temperature gradually dropped below this limit, although the pentahydrate remained liquid – it became a so-called supercooled liquid. Then, at about 41.5 °C, the substance began to heat up without the action of an external source – that is the moment it began to solidify when releasing latent heat of solidification. The temperature rose to the freezing point, and the liquid crystallized; the formation of crystals can be observed by the naked eye, as shown in Fig. 3.
Technical Notes
The degree to which thiosulphate can be supercooled depends strongly on its purity and also on the purity of the beaker and the temperature sensor itself. Any impurities can serve as crystallization nuclei and the effect of supercooling can be significantly reduced. But the truth is that for sodium thiosulphate pentahydrate, this phenomenon is so strong that you can observe supercooling under not-so-ideal conditions.
If the temperature drops below 30 °C and the substance still does not show signs of solidification, 5 minutes before the end of the measurement, it is advisable to drop a few thiosuplhate crystals into the smaller beaker, thus initiating the solidification process. Otherwise, the time set on the horizontal axis may not be long enough to record the solidification.
After the measurement, the smaller beaker is filled with solid thiosulphate. Due to its relatively low melting point, you can dispose it by rinsing the beaker with hot tap water. You can also reheat the beaker over the flame, but once used, thiosulphate has proven worthless for further measurements – even at higher temperatures, there are solid “filaments” that represent significant distortions to homogenity that do not disappear during melting.
Pedagogical Notes
As can be seen from the sample graph, the whole experiment takes quite a long time: up to 30 minutes; in addition, it is necessary to add the initial time required for melting the pentahydrate. It is appropriate to start your lesson with this experiment so that it is possible to finish it during the lesson.
It is not necessary to pay attention to the experiment while the pentahydrate is cooling down. At the end of the lesson, however, you should have a few minutes to comment on the measured graph.
It is interesting to note that other supercooled liquid – sodium acetate trihydrate – is used as a filling of warm pads (Fig. 4). This substance remains liquid at room temperature, although its freezing point is 58 °C. Crystallization of the trihydrate which leads to heating it to the freezing point is initiated by crushing the plate which is sealed inside the pad.